Abstract
Background: Measurable residual disease (MRD) detected by multiparameter flow cytometry (MFC) and/or by NPM1 gene mutations reverse transcriptase polymerase chain reaction (RT-PCR) after two cycles of high-dose induction chemotherapy has strong prognostic value and predicts relapse when applied to patients with acute myeloid leukemia (AML) in complete remission (CR). The Dutch-Belgian Hemato-Oncology Cooperative Group and Swiss Group for Clinical Cancer Research (HOVON-SAKK) AML132 trial used the MRD result at this time-point to guide post-remission consolidation treatment in ELN-2017 intermediate-risk patients. MRD-positive patients were advised to receive an allogeneic stem cell transplantation (allo-SCT) as consolidation therapy, because of the additional graft-versus-leukemia effect. On the other hand, patients in CR with a negative MRD status before transplant were advised to receive a non-allo-SCT consolidation therapy due to a relatively low risk of relapse. This could either be continuation with chemotherapy or high-dose chemotherapy with autologous stem cell transplantation (auto-SCT). In the ELN-2017 intermediate-risk group of patients treated in this trial, no significant difference in relapse-free survival between MRD-positive and MRD-negative patients was observed indicative of successful guidance. However, the exact effect of MRD-guidance was not yet fully elucidated.
Aim: Here, we investigate the impact of MRD-guidance for ELN-2017 intermediate-risk patients by comparing the MRD-guided AML132 cohort with a matched MRD-unguided group derived from previous HOVON trials using a propensity score match (PSM) analysis. We consider this to be a valuable alternative to a randomized controlled trial for which no data are available.
Methods: The MRD-unguided cohort was selected from intermediate-risk patients in CR with MRD measurement after induction therapy of four HOVON-SAKK phase 2/3 trials (AML42A, AML81, AML92 and AML102). Via PSM, these patients were matched to the AML132 MRD-guided patients using six baseline variables that are associated with survival (age, white blood cell count at diagnosis, WHO-classification, karyotype, NPM1 status and FLT3-ITD status). We used the 'nearest neighbor' matching technique with a caliper (maximum distance between cases) of 0.25. Survival differences in event-free survival (EFS) and overall survival (OS) between matched cohorts were assessed using Cox regression analysis and visualized via Kaplan-Meier curves.
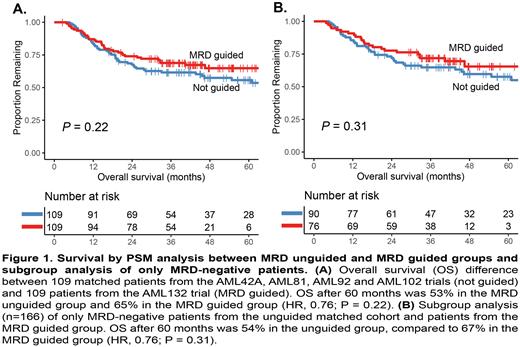
Results: The PSM analysis resulted in 109 matches with similar patient characteristics and an overall standardized mean difference of 0.11. All clinical features between the AML132 patients and matched control group were similar, except for cytogenetic classification and proportion of MRD-positive patients after cycle II. EFS (hazard ratio (HR), 0.92; P= 0.66) and OS (HR, 0.76; P= 0.22; Figure 1A) were comparable between the AML132 MRD-guided group and the historical control cohort. Subgroup analysis was performed for MRD-negative patients, since preferred consolidation treatment only changed for these patients. Percentage of MRD-negative patients receiving a non-allo-SCT consolidation therapy increased from 39.0% in the unguided cohort to 51.4% in the MRD-guided cohort. The EFS after three years (HR, 0.91; P= 0.67) and OS after five years (HR, 0.76; P= 0.31; Figure 1B) were not significantly different between unguided and guided MRD-negative patients, despite the relatively less intensive treatment in the latter. Limitation of the PSM approach is the improvement of outcome of the different trials over the years, which may be of influence on the analyses. Nevertheless, analyses within the AML132 trial showed that 33 allo-SCT could be averted and 12 postponed for MRD-negative patients without decrease in survival.
Conclusion: These results underline that guiding post-induction therapy based on MRD result does increase the number of non-allo-SCT consolidation treatment choices for intermediate-risk patients while maintaining similar EFS and OS compared to unguided patients. Therefore, non-allo-SCT seems to be justified for intermediate-risk MRD-negative patients.
Disclosures
Gjertsen:Pfizer Inc: Consultancy; Alden Cancer Therapy: Current holder of stock options in a privately-held company; KinN Therapeutics: Current holder of stock options in a privately-held company; Novartis: Consultancy; BerGenBio: Consultancy. Griskevicius:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees. Janssen:Roche: Speakers Bureau; Ellipses Pharma: Research Funding; Avillion: Research Funding; Glycomimetics: Research Funding; Uppsala County Council: Research Funding; Pfizer: Consultancy; Incyte Biosciences Benelux BV: Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding. Manz:CDR-Life Inc: Consultancy, Current holder of stock options in a privately-held company; University of Zurich: Patents & Royalties: CD117xCD3 TEA. Porkka:Pfizer: Honoraria; Celgene/Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Novartis: Honoraria; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria; Astellas: Honoraria; AbbVie: Honoraria. Lowenberg:AbbVie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio Inc.: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Clear Creek Bio: Consultancy, Honoraria; F. Hoffmann La Roche: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio Inc.: Membership on an entity's Board of Directors or advisory committees. Ossenkoppele:Pfizer: Consultancy; BMS/Celgene: Consultancy, Honoraria; Janssen: Consultancy; AGIOS: Consultancy, Honoraria; Amgen: Consultancy; Gilead: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy; Merus: Consultancy; Novartis: Consultancy, Honoraria, Research Funding. Cloos:Merus: Other: MRD assessments, Research Funding; Astellas: Speakers Bureau; Novartis: Consultancy, Other: MRD assessments, Research Funding; Janssen: Research Funding; Genentech: Research Funding; DC-One: Other: MRD assessments, Research Funding; Helsinn: Other: MRD assessments; Navigate: Patents & Royalties: Royalties for MRD analyses; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal